Traditional pharmacologic approaches had identified several classes of xenobiotics that elicited characteristic biological effects in vivo but that lacked defined molecular mechanisms of action.
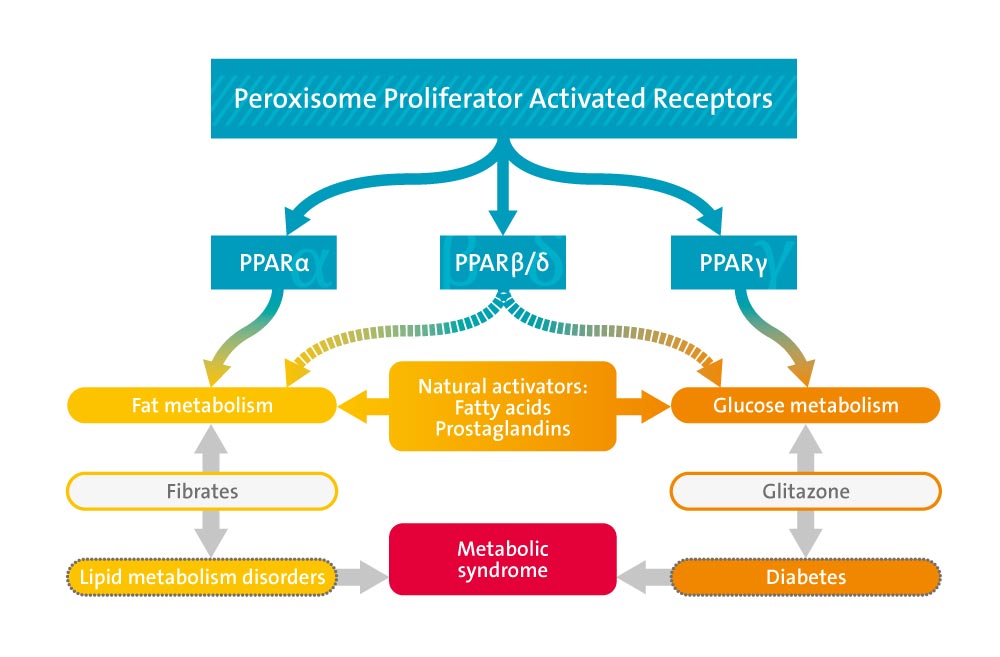
Among these xenobiotics were the peroxisome proliferators, the thiazolidinediones (TZDs), and a set of compounds that induced the expression of cytochrome P450 (CYP) 3A genes and promoted the metabolism of other xenobiotics. All three classes of xenobiotics are now known to exert their actions through activation of orphan members of the nuclear receptor family of ligand-activated transcription factors.
Peroxisome proliferators are a diverse group of amphipathic acids that include the fibrate class of triglyceride- and cholesterol-lowering drugs. TZDs sensitize tissues such as skeletal muscle, liver, and adipose to the actions of insulin and lower glucose and lipid levels in type 2 diabetics. The peroxisome proliferators and TZDs are now known to mediate their effects through the peroxisome proliferator-activated receptors (PPARs) alpha and gamma, respectively. The activities of these compounds established the PPARs as key regulators of glucose and lipid homeostasis.
We and others have recently shown that various naturally occurring fatty acids and eicosanoids serve as PPAR ligands, suggesting a novel regulatory mechanism whereby dietary lipids and their metabolites can regulate gene transcription and impact overall energy balance.
The third class of xenobiotics we have studied induces the expression of CYP3A genes, mono-oxygenases responsible for the metabolism of natural steroids as well as a variety of xenobiotics, including > 60% of all drugs.
We have recently shown that compounds that induce CYP3A gene expression do so through activation of novel orphan receptors, termed the pregnane X receptors (PXRs). Many of the PXR activators are widely used drugs such as dexamethasone, lovastatin, and rifampicin, whose induction of CYP3A levels causes them to promote the metabolism of other drugs, often with adverse consequences.
Thus, the finding that the PXRs regulate CYP3A gene expression provides a basis for the efficient identification and elimination of candidate drugs that will interact with other medicines. Searches for natural ligands have revealed that the PXRs are activated by C21 steroids, including pregnenolone and progesterone, suggesting that these orphan receptors define a novel steroid hormone signaling pathway. In sum, work from our laboratories and others has demonstrated that peroxisome proliferators, TZDs, and inducers of CYP3A gene expression exert their biological actions through the activation of orphan nuclear receptors.
These findings provide insights into new endocrine signaling pathways and have important implications for the discovery of safer and more efficacious drugs for the treatment of a variety of diseases.