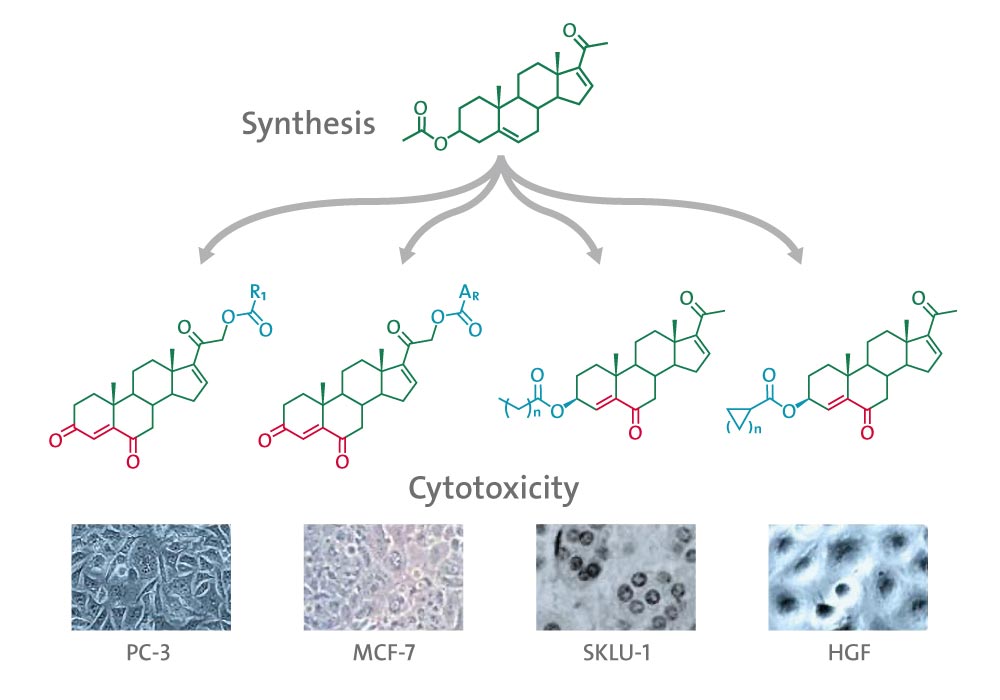
Four series of pregnenolone derivatives having one or two α,β-unsaturated carbonyls and an ester moiety at C-21 or C-3 were synthetized to compare their cytotoxicity effect.
The final compounds were evaluated on three human cancer cell lines: PC-3 (prostate cancer), MCF-7 (breast cancer), SKLU-1 (lung cancer) and a noncancerous cell line HGF (human gingival fibroblast).
Two steroids with a 4-fluorinated benzoic acid ester at C-21 were the most active against lung cancer cell line with IC50 of 13.1 ± 1.2 and 12.8 ± 0.5 μM and showed a low percentage of cytotoxicity for noncancerous cells (27.63 ± 2.3 and 18.39 ± 1.2 % in the screening at 50 μM).