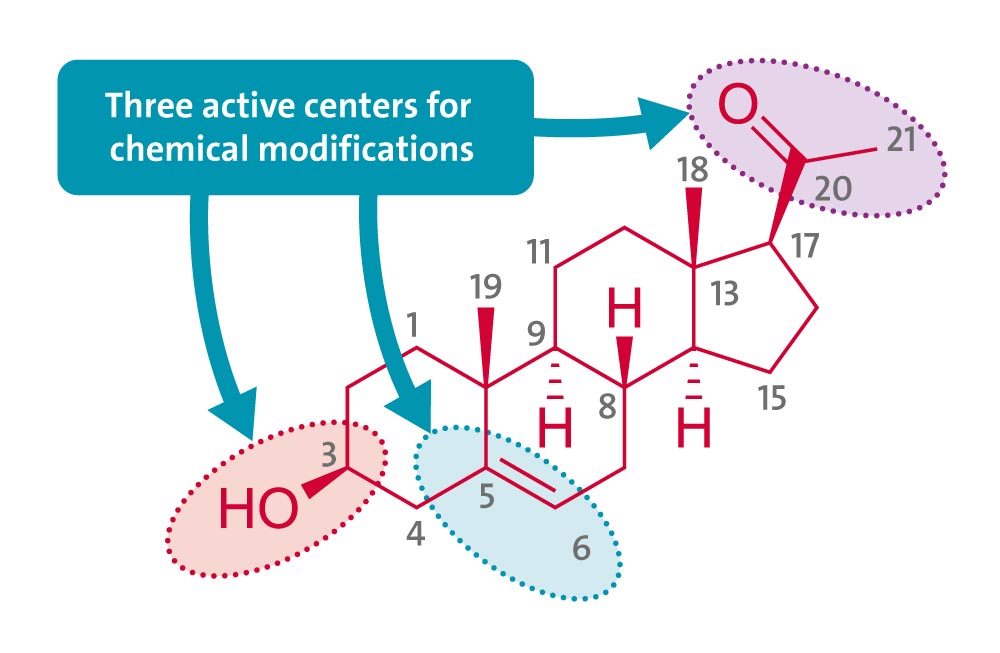
The structural modifications of pregnenolone have been described via the introduction of heterocyclic moieties at C-17 position by limiting the acyl group.
Novel heterocyclic analogues of pregnenolone have been synthesized by using Friedlander and Claisen-Schmidt reactions, and the synthesized compounds were evaluated for their osteogenic activity.
Among the synthesized derivatives, four compounds showed significantly increased ALP activity. Among all four active compounds, the novel compound 3a has shown significant bone matrix mineralization and mRNA expressions of osteogenic marker genes, BMP2, RUNX-2 and OCN at 1pM concentration.